BUSINESS
CDMO
GenixCure CDMO
-
Our CMO service facilitates customers’ preclinical programs of AAV gene therapy. Sponsors or academic researchers can be supported by Genixcure’s customized service from design to manufacturing of various serotypes. We offer quasi-GMP grade vectors based on optimized process from plasmids design to QA of produce
• Process development : we offer customized process development for manufacturing & purification of AAV and plasmid DNA and transfer SOP-driven preclinical CMO
• Development of Analysis Method : Development of QC analysis method equivalent to GMP grade
• Customized plasmid & AAV production by cell types and scale
-
1 AAV Design
Plasmid / AAV
Upon request of customers, we design to find an optimized GOI plasmid to certain target cell/tissue and assist customer’s serotype selection in accordance with MoA of each therapeutic program.
-
2 AAV Manufacturing & Purification
Plasmid / AAV Production Services
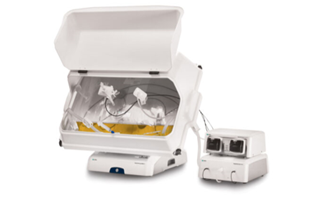
As an AAV-gene-therapy-focused biotech, Genixcure leverages its expertise and experience to professionally work as a preclinical CMO service provider
-
Equipped with its own clean room facilities
-
Production scale
• Adherent cell (cell stack → maximum 80 layers)
• Suspension cell (single use → maximum 25L)
-
Equipped with its own clean room facilities
-
3 Quality Assurance
Analysis and Quality Assurance Services
We issue CoA(Certificate of Analysis) normally including analytical assurance of followings
-
AAV (DP)
• Titation (qPCR/ddPCR)
• Full/empty ratio (TEM/qPCR and ELISA ratio analysis)
• transduction efficiency (fluorescent microscope/TCID50) -
Drug substance
• Endotoxin
• SDS-PAGE
• stability test
• HPLC analysis
• Mycoplasma test
-
AAV (DP)
Main Composition
- Production
- Refinement
- Analysis

-

Production process development
-

Customized Plasmid Production
-

Production of Customized Viruses
dquipment
Features
- Design of optimized GOI plasmid to targets and serotypes
- Both adherent and suspension platform available
-
Production scale
• Adherent cell – 150mm cell culture dish ~ cell stack (80 layers)
• Suspension cell – 1~25L scale (wave bioreactor)

-
Development of Harvest
and lysis -

Purification process optimization
-

High concentration and clarification/formulation
equipment
Features
- Harvest and analysis methods for the target
- Buffer exchange and concentration using UF/DF
- Refining process using FPLC (~25 L)

-

Characterization of Production Samples
-
Safety Analysis of Production Samples
-

COA Issuance
equipment
Features
-
Titer Analysis
• qPCR, ddPCR -
Purity analysis
• SDS-PAGE, HPLC, Full/empty ratio analysis (transmission electron microscope analysis) -
Safety Analysis
• Aseptic test, Endotoxin, Mycoplasma - Certificate of Analysis (CoA)
PROCEDURES of OUR SERVICES
-

Apply for service
-

Discussion on
Customized
production -

Confidentiality
agreement -

Process development,
sample production and
analysis of quality of
samples produced -

Transfer of CoA and
samples produced
througj consignment
- Starting from the discussion of virus scale via email, call, or F2F meeting
- Finding agreed range of characteristics analysis by customers’ use of viruses
- Finalizing customers’ request on CoA features and quantitative grade of each feature
- QA-based CoA issued and material transfer
Contact Us
-

Contact Us Online
-

Contact Us by Phone
031 - 213 - 5115