뉴스
Development of GENIXCURE, miRNA-regulated AD gene therapy
2021-01-11
DIGIST-IBS joint research product gene therapy drug 'ANL-101' obtained a U.S. patent.Improve cognitive function by simultaneously controlling multiple target proteins and strengthening synapses."I'm going to start a full-time clinical trial"

GENIXCURE will start commercialization in earnest by acquiring a U.S. patent for a new Alzheimer's dementia gene therapy. GENIXCURE is a miRNA-based new drug development company established in April 2019 by CEO Moon Hong-sung, who served as chairman of Chemon, and CEO Kim Keetae, a senior researcher at the Daegu Gyeongbuk Institute of Science and Technology (DGIST).
GENIXCURE announced on the 16th that it has recently obtained a U.S. patent for "ANL-101," a candidate for Alzheimer's dementia treatment. ANL-101 previously secured a domestic patent in 2017 and acquired a U.S. patent in three years.
ANL-101 is the product of aging-related miRNA research conducted jointly by the Korea Institute of Basic Science (IBS) Plant Life Aging Research Group (Director Nam Hong-gil, Co-inventor) and the Daegu Gyeongbuk Institute of Science and Technology (Major Researcher Kim Keetae) since 2012.
So far, there are five treatments for Alzheimer's disease, including Donepezil and Galaltamine, which are only symptoms-relieving retardants rather than basic treatment for Alzheimer's disease, and many new drug development challenges have succeeded in reducing amyloid beta protein and tau protein, but have not reached cognitive improvement.
ANL-101, patented by GENIXCURE, is a new drug candidate for a new concept gene therapy that inhibits or promotes the expression of proteins in the human body through the regulation of core miRNAs involved in aging in the human body. It is explained that Alzheimer's disease can be treated with a single administration.
In addition to various deterioration of functions due to aging, Alzheimer's disease is the result of multiple factors at the same time, said CEO Moon Hong-sung. "We can restore cognitive function to normal only through complex prescriptions that prevent the death of neurons through inflammation relief in the brain and strengthen synapses."
The company confirmed the therapeutic effect of ANL-101 in an experimental model for Alzheimer's disease that improves cognitive and learning ability to a normal level of the mouse. As a result of administering ANL-101 to Alzheimer's model mice, various target proteins were simultaneously controlled, and proteins involved in differentiation, growth, and formation of synapses, a neurotransmission zone, increased by 70%, restoring cognitive and memory. In addition, proteins involved in cell protection and energy metabolism such as anti-aging, anti-inflammatory, and antioxidant increased by 60%, confirming that nerve cell protection functions are activated by reducing amyloid beta protein and removing inflammation.
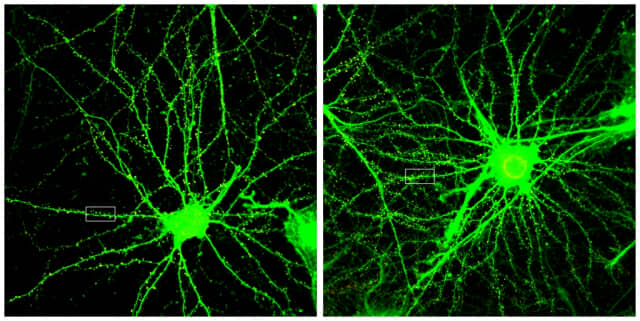
▲A microscopic photograph of increased dendritic protrusion visibility of nerve cells before/after administration of ANL-101. Provided by GENIXCURE.
In addition, as a result of animal experiments, the number of dendritic spines, which are tentacles for information reception of nerve cells, increased by about 20% in mice administered with ANL-101. This means that the number of synapses in which signals are transmitted between neurons has increased, which means that the function of nerve cells can be improved. It has been confirmed that ANL-101 is also effective in restoring cognitive function, long- and short-term memory, and improving learning function, which is the core of normalization of Alzheimer's disease, and restoring synaptic long-term reinforcement efficiency (LTP), a fundamental mechanism for restoring cognitive ability.
An GENIXCURE official said, "We will expand the indication from Alzheimer's disease to mild cognitive impairment (MCI) and severe forgetfulness based on the high cognitive function improvement effect of ANL-101, and we have completed the contract."
Meanwhile, GENIXCURE opened in April 2019 with the idea of "beautiful aging and healthy longevity." Moon Hong-sung, former chairman of the non-clinical CRO Chemon, met with Kim Keetae, a senior researcher at DGIST, and co-founded it while promoting investment and commercialization related to aging. Kim served as a research assistant professor at POSTECH and a senior researcher at DGIST in Korea after attending Seoul National University (Zoology), Texas State University (Microbiology Ph.D.) and Harvard Medical School (postdoctoral course).
GENIXCURE received technology related to degenerative brain disease, which was invented by Dr. Kim Keetae, from the Institute of Basic Science and the Daegu Gyeongbuk Institute of Science and Technology. In addition to Alzheimer's disease, the company is developing gene treatments such as Parkinson's disease, degenerative obesity and diabetes, epilepsy, and senile macular degeneration, and has applied for domestic patents based on animal experiments that prove its therapeutic effect.
- 이전글
- 이전글이 없습니다.

